Multiple Choice
Formaldehyde is a covalent compound used as a preservative for biological specimens.Which statement concerning the Lewis structure of formaldehyde,shown below,is FALSE? 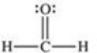
A) Four electrons are being shared between the carbon and oxygen atoms.
B) The oxygen atom has four valence electrons that are not being shared.
C) The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D) The hydrogen atoms have incomplete valence shells.
E) There are a total of 12 valence electrons in formaldehyde.
Correct Answer:

Verified
Correct Answer:
Verified
Q67: Which of the following is an essential
Q68: Which statement about compounds is FALSE?<br>A)Compounds consist
Q69: Assuming reactions between the following pairs of
Q70: What is the proper name for Mg(CN)<sub>2</sub>?<br>A)manganese
Q71: Ionic solids are amorphous.
Q73: How many total valence electrons are in
Q74: Which combination of atoms is most likely
Q75: Which Lewis symbol for an ion is
Q76: What is the formula of the ionic
Q77: In determining properties such as solubility,melting point,and