Multiple Choice
What is wrong with the Lewis structure shown for sulfur trioxide,SO3? 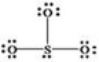
A) Oxygen,not sulfur,should be the central atom.
B) Sulfur should have 10 electrons around it instead of 8,to show its expanded octet.
C) The structure shows 26 valence electrons,but there should only be 24.
D) There are too many bonding electrons shown.
E) The structure should show each oxygen atom with a double bond to the sulfur atom.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: What does it mean if an atom
Q33: Which of the following Lewis structures has
Q34: Which of the following bonds would be
Q35: The drug "lithium" is often prescribed to
Q36: What is the formula of the ionic
Q38: Assuming reactions between the following pairs of
Q39: What are the two principal classes of
Q40: There are three atoms of iodine represented
Q41: The polyatomic ion known as the diphosphate
Q42: How many nonbonding electrons are in CH<sub>4</sub>?<br>A)0<br>B)1<br>C)2<br>D)3<br>E)8