Multiple Choice
How many bonding electrons are present in the Lewis structure for the bicarbonate ion,shown below? 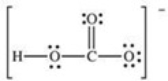
A) 4
B) 5
C) 8
D) 10
E) 24
Correct Answer:

Verified
Correct Answer:
Verified
Q20: How many bonding electrons are in CO<sub>2</sub>?<br>A)1<br>B)2<br>C)3<br>D)4<br>E)8
Q21: A double bond between two atoms,A and
Q22: In the molecule BeF<sub>2</sub>,the beryllium atom is
Q23: Which formula represents ammonia?<br>A)Al<sub>3</sub><br>B)AlH<sub>3</sub><br>C)NH<sub>4</sub>+<br>D)NH<sub>3</sub><br>E)AN<sub>4</sub>
Q24: What is the name of Fe<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub> in
Q26: Baking soda consists of the ionic compound
Q27: What is the Lewis structure of methanethiol,CH<sub>3</sub>SH?<br>A)<br><img
Q28: What is the formula of the sulfate
Q29: In the molecule AX<sub>2</sub>,the central atom A
Q30: How many dots are present in the