Multiple Choice
According to the VSEPR theory,what is the geometry (shape) around the phosphorus atom in the Lewis structure shown below? 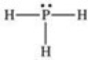
A) linear
B) bent (angular)
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q79: In Lewis symbols,the chemical symbol of an
Q80: What is the name of the compound
Q81: Which statement is TRUE concerning the solubility
Q82: What is the molecular geometry of CO<sub>2</sub>?<br>A)linear<br>B)trigonal
Q83: Which one of the following is NOT
Q85: What kind of bond results when electron
Q86: Predict the formula of the compound formed
Q87: What kind of bonding exists in substances
Q88: The NO molecule can never satisfy the
Q89: In what region on the periodic table