Multiple Choice
Hydrogen gas is heated to the point at which there are electrons at atomic energy levels up to the n = 3 level. When electrons return to the ground state, what possible emission lines from which spectral sequences will result? See Figure 4-11. 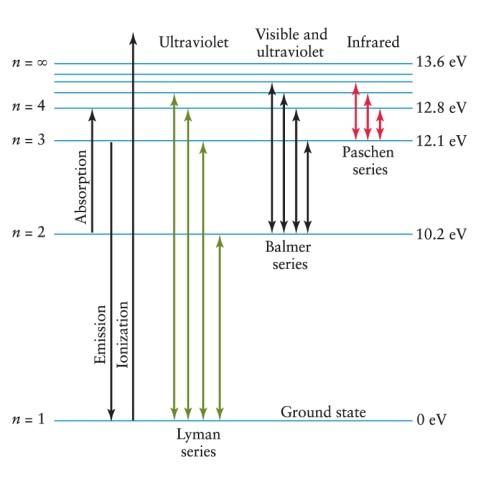
A) Paschen (IR) , Balmer (visible) , and Lyman (UV) series
B) Lyman (UV) series only
C) Balmer (visible) series only
D) Balmer (visible) and Lyman (UV) series
Correct Answer:

Verified
Correct Answer:
Verified
Q23: A hydrogen atom in a low-density, hot
Q24: When a blackbody is heated to
Q25: Emission spectra from interstellar gas clouds glow
Q26: If one neutron is added to a
Q27: The temperature of the surface of the
Q29: A source emitting waves of constant wavelength
Q30: The basic makeup of an atom is<br>A)
Q31: Considering the oxygen isotopes <sup>15</sup>O and <sup>16</sup>O,
Q32: Which one of these processes results in
Q33: Light that originates in hydrogen atoms in