Multiple Choice
An electron is in the n = 3 energy level in a hydrogen atom. To ionize this atom, it is necessary for the electron to gain a minimum of how much energy? See Figure 4-11. 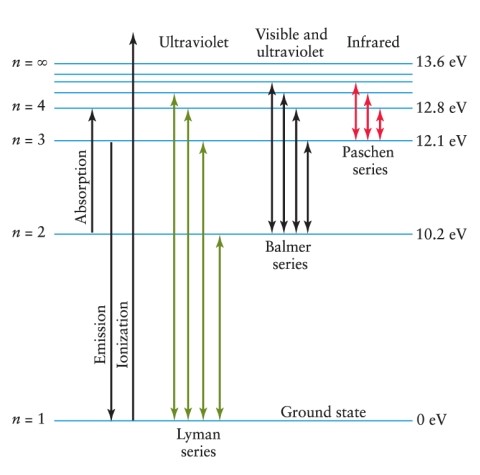
A) 1.5 eV
B) 4.5 eV
C) 12.1 eV
D) 13.6 eV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q126: A scientist reports that his measurement of
Q127: Carbon-14 (<sup>14</sup>C) has a half-life of approximately
Q128: When a solid body (or a dense
Q129: Where and by what technique was the
Q130: An electron is in the n =
Q132: The diameter of the nucleus of a
Q133: Radiant energy shines on a blackbody, raising
Q134: The important breakthrough in theoretical physics
Q135: Of the four combinations of particles that
Q136: The force that holds the atomic nucleus