Multiple Choice
Which of the following Lewis structures represent resonance forms of ozone, O3? 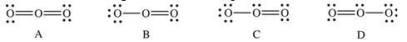
A) A and B
B) B and C
C) C and D
D) A and C
E) A and D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: What is the name of the ion
Q20: How many bonding electrons are in CO<sub>2</sub>?<br>A)1<br>B)2<br>C)3<br>D)4<br>E)8
Q44: Because the C-H bond in methane is
Q54: What statement about the ammonia molecule,NH<sub>3</sub>,is FALSE?<br>A)The
Q59: A molecule of deoxyribose,an essential part of
Q68: Which statement about compounds is FALSE?<br>A)Compounds consist
Q85: Vehicle airbags inflate when the ionic compound
Q87: Draw the Lewis structures of F<sub>2</sub>, O<sub>2</sub>
Q88: In the molecule AX<sub>2</sub>, the central atom
Q91: Which compound contains a central atom with