Short Answer
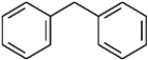
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
-_______ 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Predict the splitting of each of the
Q19: Which of the following compounds gives a
Q20: For each of the compounds below tell
Q21: How many sets of equivalent protons are
Q22: Which of the protons in the
Q24: Identify the compound (C<sub>8</sub>H<sub>10</sub>O<sub>2</sub>) that gives the
Q25: What is the splitting of the signal
Q26: Which feature in the <sup>1</sup>H NMR spectrum
Q27: Which feature in the <sup>1</sup>H NMR spectrum
Q28: Which of the protons in the following