Short Answer
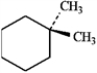
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
-_______ 
Correct Answer:

Verified
Correct Answer:
Verified
Q79: The following compound will have a <sup>1</sup>H
Q80: Identify the most appropriate intensity maintained for
Q81: Identify the compound (C<sub>4</sub>H<sub>8</sub>O) that gives the
Q82: How many signals appear in proton-decoupled <sup>13</sup>C
Q83: Which of the protons in the
Q85: Identify the compound (C<sub>4</sub>H<sub>6</sub>O<sub>2</sub>) that gives the
Q86: Identify the compound (C<sub>3</sub>H<sub>7</sub>Cl) that gives the
Q87: The chemical shift of the protons of
Q88: Identify the methyl hydrogen with the
Q89: How many signals appear in proton-decoupled <sup>13</sup>C