Essay
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 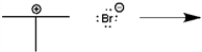
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: Match the types of reactions with the
Q79: Use curved arrows to show the movement
Q80: What is the approximate pK<sub>a</sub> value of
Q81: Provide the equation that relates the
Q82: Which of the following is a Lewis
Q84: Which of the following is the strongest
Q85: Which atom in the following structure is
Q86: Why are phenols more acidic than alcohols?
Q87: Which sets of curved arrows accounts for
Q88: Which of the following is present in