Multiple Choice
Which of the following best represents the shape of the 2s atomic orbital of carbon? 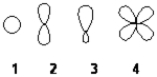
A) 1
B) 2
C) 3
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: Which atomic orbitals overlap to form the
Q102: Circle all of the sp<sup>2</sup> hybridized atoms
Q103: In the given ion given, what is
Q104: Which of the following resonance structures is
Q105: Which of the following compounds is a
Q107: Overlap of the two atomic orbitals as
Q108: The formal charges in the complex should
Q109: Which of the following statements is not
Q110: Which of the following is a primary
Q111: What is the approximate H−C−O bond angle