True/False
The hybridization on the numbered carbon atoms in the following compound would be Carbon 1 sp3 and Carbon 2 sp2. 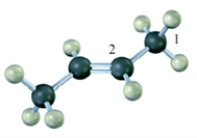
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: How many electrons can the shell with
Q54: Which of the following is trigonal planar?<br>A)
Q55: Consider the following structural formula. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7078/.jpg"
Q56: Convert the following structure into a bond-line
Q57: How many electrons are there in the
Q59: Draw bond-line structures of all of the
Q60: Which of the following elements has the
Q61: Which of the following molecules is not
Q62: What is the approximate value of the
Q63: Which of the following is a secondary