Multiple Choice
The compound below is classified as what type of compound? 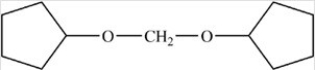
A) An acetal
B) A hemiacetal
C) An ether
D) A cyclic acetal
E) An acetal and an ether
Correct Answer:

Verified
Correct Answer:
Verified
Q71: Which compound has the highest boiling point?<br>A)CH<sub>3</sub>(CH<sub>2</sub>)<sub>5</sub>CH<sub>3</sub><br>B)CH<sub>3</sub>(CH<sub>2</sub>)<sub>4</sub>CHO<br>C)CH<sub>3</sub>(CH<sub>2</sub>)<sub>4</sub>CH<sub>2</sub>OH<br>D)All
Q72: _ is the simplest aldehyde.
Q73: Which compound has the greatest solubility in
Q74: The reaction below illustrates oxidation by Tollens
Q75: (CH<sub>3</sub>)<sub>2</sub>CHCOCH<sub>3</sub> has a lower boiling point than
Q77: Aldehydes and ketones have _ (higher/lower)boiling points
Q78: Which compound(s)would give a positive Tollens test?<br>A)Alcohols<br>B)Aldehydes<br>C)Ketones<br>D)Carboxylic
Q79: What acetal is formed when the compound
Q80: Deoxyribose is a building block of DNA.
Q81: The reaction below illustrates oxidation of an