Multiple Choice
Carvone,shown below,is a chiral compound that exists as a pair of enantiomers. One enantiomer has the odor of caraway,and the other enantiomer has the odor of spearmint. Identify the chirality center(s) in carvone. 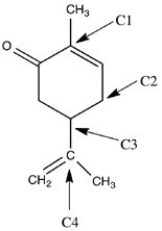
A) C1
B) C2
C) C3
D) C4
E) C1 and C3
Correct Answer:

Verified
Correct Answer:
Verified
Q81: Two enantiomers rotate plane-polarized light to an
Q82: Another term in common use for a
Q83: The Fischer projection shown on the right
Q84: cis-2-butene and trans-2-butene are enantiomers.
Q85: Diastereomers are stereoisomers that are _.<br>A)mirror images
Q87: All molecules that have carbons with a
Q88: Which compound has the most stereoisomers?<br>A) <img
Q89: Which object is chiral?<br>A)A sock<br>B)A shoe<br>C)A baseball<br>D)A
Q90: Ethambutol (structure shown)is a bacteriostatic antimycobacterial drug
Q91: The Fischer projection shown on the right