Multiple Choice
What are the bond angles in the structure shown below? 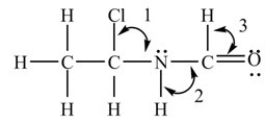
A) Angle 1 = 109.5°,Angle 2 = 109.5°,and Angle 3 = 109.5°
B) Angle 1 = 109.5°,Angle 2 = 120°,and Angle 3 = 120°
C) Angle 1 = 109.5°,Angle 2 = 90°,and Angle 3 = ~109.5°
D) Angle 1 = 109.5°,Angle 2 = ~109.5°,and Angle 3 = 120°
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Which structure is not possible?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7327/.jpg"
Q43: Which formula represents an inorganic compound?<br>A)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>B)CH<sub>3</sub>NHCH<sub>2</sub>CH<sub>3</sub><br>C)ClCCCl<br>D)CaCl<sub>2</sub>
Q44: In terms of type of compound,the compound
Q45: A functional group is an atom or
Q46: Vitamin E (structure shown)is a (fat/water)_-soluble vitamin.
Q48: What is the bond angle associated with
Q49: What is the shape around each carbon
Q50: The compound below contains no polar bonds.
Q51: In order to complete the structure of
Q52: Which structure has all of the hydrogens