Multiple Choice
Three of the four structures below represent unstable organic compounds that are not likely to exist because they violate the octet rule. Which one of the four structures represents a stable organic compound that is likely to exist?
A) 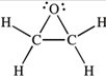
B) 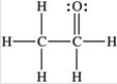
C) 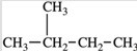
D) 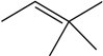
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: Vitamin C is a water-soluble vitamin. <img
Q75: CH<sub>2</sub>F<sub>2</sub> is a nonpolar molecule.
Q76: MTBE is soluble in both gasoline and
Q77: The compound below is an example of
Q78: The molecule drawn below is (polar/nonpolar)_. <img
Q80: In order to complete the structure below,_
Q81: Which represents a condensed structure for a
Q82: The molecule CH<sub>3</sub>SH is what type of
Q83: Which element is not a common heteroatom
Q84: A C atom surrounded by three atoms