Multiple Choice
Butane,CH3CH2CH2CH3,has the structure shown below. What is the strongest type of intermolecular force that exists between two butane molecules? 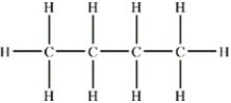
A) London dispersion forces
B) Hydrogen bonding
C) Temporary dipole interactions
D) Dipole-dipole interactions
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Energy is released when a less organized
Q32: A sample of neon gas has a
Q33: A birthday balloon contains helium at a
Q34: If 10.0 g of Ne and 10.0
Q35: A scuba diver typically begins a dive
Q37: A scuba diver typically begins a dive
Q38: Gay-Lussac's law relates the _ and temperature
Q39: A 54.2 L sample of gas at
Q40: Three of the four phase changes below
Q41: Which assumption is NOT part of the