Multiple Choice
Consider the two liquids A and B shown in closed containers. Which liquid has the higher vapor pressure? 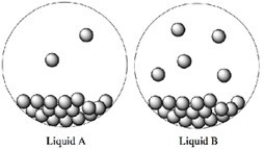
A) Liquid A
B) Liquid B
C) Both Liquids A and B have equal vapor pressures.
D) Not enough information is given.
Correct Answer:

Verified
Correct Answer:
Verified
Q75: The type(s)of intermolecular forces exhibited by hydrogen
Q76: The energy required to break up the
Q77: An aerosol can has a pressure of
Q78: How many moles of gas are contained
Q79: Polypropylene,a typical plastic,is an example of an
Q81: Graphite is an example of a _
Q82: Which molecule(s)exhibit hydrogen bonding?<br>A)CH<sub>4</sub><br>B)CHCl<sub>3</sub><br>C)NF<sub>3</sub><br>D)HF<br>E)All of the molecules
Q83: Which compound has the lowest boiling point?<br>A)
Q84: Sarin,a nerve gas once used as a
Q85: London dispersion forces are also referred to