Multiple Choice
The molecular art depicts the following reversible reaction at equilibrium: CO(g) + Cl2(g)  COCl2(g) . What can be inferred about the equilibrium constant,K,for this reaction?
COCl2(g) . What can be inferred about the equilibrium constant,K,for this reaction? 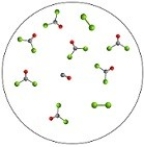
A) K < 1
B) K ~ 1
C) K > 1
D) K = 0
Correct Answer:

Verified
Correct Answer:
Verified
Q22: Which statement about catalysts is NOT true?<br>A)A
Q23: In order to cause an endothermic equilibrium
Q24: A peanut butter and jelly sandwich contains
Q25: A chemical reaction releases 55.2 kcal. How
Q26: Which K value below is consistent with
Q28: Which of the following is NOT a
Q29: Consider the combustion reaction of propane: C<sub>3</sub>H<sub>8</sub>(g)+
Q30: When the equilibrium constant for a reversible
Q31: Which value (if any)in each pair corresponds
Q32: Consider the following reversible reaction at equilibrium: