Multiple Choice
Consider the reversible reaction at equilibrium: N2(g) + O2(g) 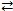 2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
A) The concentration of O2(g) increases
B) The concentration of O2(g) decreases
C) The concentration of NO(g) increases
D) The equilibrium system shifts to the right
Correct Answer:

Verified
Correct Answer:
Verified
Q85: Changes in potential energy occur in chemical
Q86: One step in the metabolism of glucose
Q87: Once equilibrium is reached in a chemical
Q88: A reversible reaction in which K =
Q89: The ΔH for the reaction depicted by
Q91: Which of the following is ALWAYS necessary
Q92: When reactants can come together and form
Q93: Heat is absorbed in an _ reaction.
Q94: Bond breaking is endothermic.
Q95: Le Châtelier's principle is a general rule