True/False
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 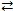 COCl2(g). If the pressure inside the reaction vessel is increased,the equilibrium will shift to the left.
COCl2(g). If the pressure inside the reaction vessel is increased,the equilibrium will shift to the left.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Breaking a chemical bond requires energy.
Q16: As a general rule,a reaction rate _
Q17: The rusting of iron is described by
Q18: Exothermic reactions involve the formation of products
Q19: The law of conservation of energy states
Q21: Consider the following reversible reaction at equilibrium:
Q22: Which statement about catalysts is NOT true?<br>A)A
Q23: In order to cause an endothermic equilibrium
Q24: A peanut butter and jelly sandwich contains
Q25: A chemical reaction releases 55.2 kcal. How