Multiple Choice
How many nonbonded electron pairs are in the Lewis structure below? 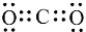
A) 2
B) 4
C) 6
D) 8
E) 16
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Which of the following compounds is classified
Q13: Every atom must have an octet of
Q14: Which of the statements concerning chemical bonds
Q15: Ethane (C<sub>2</sub>H<sub>6</sub>)is a polar molecule.
Q16: A resonance hybrid is a composite of
Q18: In Lewis structures,fluorine atoms generally do not
Q19: A molecule that contains only one polar
Q20: Phosphorus usually forms two covalent bonds in
Q21: Double bonds and triple bonds are never
Q22: Carbon tetrachloride has _ valence electrons.<br>A)4 (four)<br>B)8