Multiple Choice
Predict the bond angles around the carbon atom in the structure of carbonic acid shown below. Don't forget to draw in lone pairs where needed to give octets. 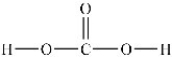
A) 180°
B) 120°
C) 109.5°
D) 90°
E) 60°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Identify the diatomic element from the given
Q2: How many valence electrons are in a
Q3: Considering the electronegativity values indicated for each
Q4: The molecule below is a polar molecule.
Q5: A double bond consists of four electrons
Q7: Rank the atoms Br,Cl,and F in order
Q8: What is the chemical formula for dinitrogen
Q9: The chemical formula for phosphorus pentachloride is
Q10: How many lone pairs of electrons are
Q11: The molecular shape around the boron atom