Multiple Choice
Which atom(s) in the structure below has(have) a partial negative charge (δ-) ? 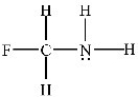
A) Carbon
B) Fluorine
C) Hydrogen
D) Nitrogen
E) Nitrogen and fluorine
Correct Answer:

Verified
Correct Answer:
Verified
Q46: How many total valence electrons does the
Q47: Covalent bonds result from the _ electrons
Q48: Bonding is the joining of two atoms
Q49: How many lone pairs of electrons need
Q50: Electronegativity _ down a column of the
Q52: Which Lewis structure is incorrect?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7327/.jpg"
Q53: What is another name for an unshared
Q54: Rank the atoms Br,Cl,and K in order
Q55: Which choice would be named dinitrogen pentoxide?<br>A)N<sub>2</sub>O<sub>5</sub><br>B)N<sub>5</sub>O<sub>2</sub><br>C)N<sub>3</sub>O<sub>2</sub><br>D)N<sub>2</sub>O<sub>3</sub><br>E)NO
Q56: To represent the three-dimensional geometry of a