True/False
The structures shown below are resonance structures of sulfur dioxide. 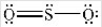 and
and 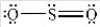
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q89: Which compound has the greatest number of
Q90: Estimate the bond angles around the sulfur
Q91: The symbol δ<sup>-</sup> is given to the
Q92: In general,a _ bond will be one
Q93: Which atom has the lowest electronegativity?<br>A)Al<br>B)S<br>C)Se<br>D)Rb<br>E)F
Q95: The covalent bond between chlorine and iodine
Q96: In the Lewis structure of a molecule,oxygen
Q97: What is the chemical formula for the
Q98: Aspartic acid is an amino acid used
Q99: Which bond has the polarity incorrectly labeled?<br>A).<sup>δ+</sup>