Multiple Choice
The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.Which of the following chemical equations represents the reaction that occurs when OH- is added to the HF/F- buffer? 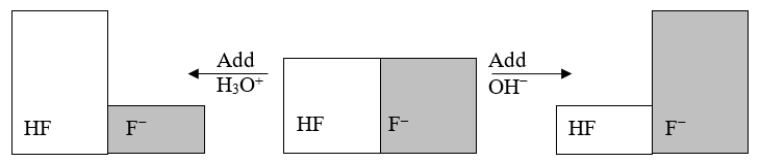
A) HF + H2O ⇌ F- + H3O+
B) F- + H2O ⇌ F- + OH-
C) HF + OH- ⇌ F- + H2O
D) F- + 2 OH- ⇌ HF + O2
E) F- + OH- ⇌ HOF
Correct Answer:

Verified
Correct Answer:
Verified
Q73: Which pH range is referred to as
Q74: Consider a buffer solution containing CH<sub>3</sub>COO<sup>-</sup>Na<sup>+</sup> and
Q75: Which of the following reactions illustrate the
Q76: What is the pH of a solution
Q77: A weak acid is also a _
Q78: Which acid is NOT a strong acid?<br>A)
Q79: The neutralization reaction of potassium hydrogen carbonate
Q80: Which statement BEST describes what it means
Q82: How do strong and weak acids differ?<br>A)
Q83: The following reaction is a reversible reaction.Which