Multiple Choice
Which statement BEST describes the following reaction? 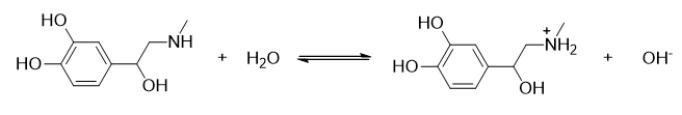
A) This is the reaction of a strong acid.
B) This is the reaction of a weak acid.
C) This reaction is the dissociation of a strong base.
D) This is the reaction of a weak base.
E) This is not an acid-base reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Which would be the BEST treatment of
Q67: What is the pH of a solution
Q68: The concentration of H<sub>3</sub>O<sup>+</sup> in a solution
Q69: What mole ratio of NaOH to H<sub>2</sub>SO<sub>4</sub>
Q70: The following figure illustrates the action of
Q72: The boxed species in the following reaction
Q73: Which pH range is referred to as
Q74: Consider a buffer solution containing CH<sub>3</sub>COO<sup>-</sup>Na<sup>+</sup> and
Q75: Which of the following reactions illustrate the
Q76: What is the pH of a solution