Multiple Choice
A gas having a volume of 11.2 L has added to it 0.5 mol of gas at the same temperature and pressure.The final volume is 22.4 L, again at the same temperature and pressure.Which relationship is used to determine the number of moles of gas originally?
A) 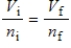
B) 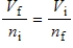
C) 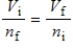
D) 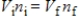
E) There is no relationship between the moles of air and the volume of air.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Henry's constant for halothane, an anesthetic, is
Q26: What happens to acetone when it is
Q27: You have a balloon containing 1.4 L
Q28: A gas having a volume of 11.2
Q29: Which of the following properties does NOT
Q31: How many calories of heat are required
Q32: On a cool morning (12 °C), a
Q33: Which statement about the density of gases
Q34: Which compound has the lowest boiling point?<br>A)
Q35: What change of phase is represented by