Multiple Choice
Each of the following figures represents a reaction.Which reaction(s) has/have the slowest rate? 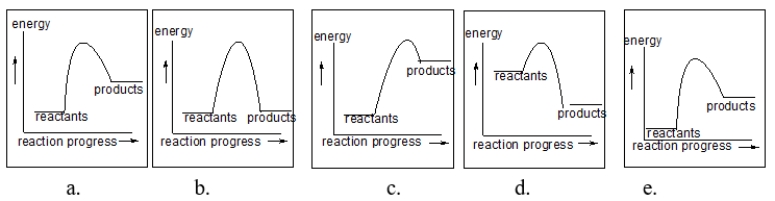
A) a and b
B) a, b, c, d and e
C) all but d
D) b only
E) b and e
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: If a reaction has a ΔH<sub>rxn</sub> >
Q12: If a reaction has a ΔH<sub>rxn</sub> >
Q13: Increasing the temperature of a reaction causes
Q14: The reaction of water with ammonia is
Q15: Carbohydrates and fats are sources of energy
Q17: A hot dog contains 151 Calories.How many
Q18: Select the TRUE statement concerning a reaction
Q19: How is the joule related to the
Q21: How would this diagram be different if
Q45: Combustion reactions are _ because products of