Multiple Choice
The ΔH for the reaction described by the energy diagram below is 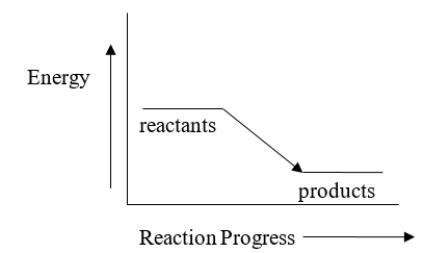
A) very large.
B) greater than zero but not necessarily very large.
C) exactly zero.
D) less than zero.
E) It is not possible to predict anything about the ΔH of this equation.
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Which statement best describes the relationship between
Q29: A spirometer is used in indirect calorimetry.What
Q30: The reaction between acetic acid (CH<sub>3</sub>COOH)and methanol
Q31: Which statement describes reaction rate?<br>A) Reaction rate
Q32: An exothermic reaction is one that:<br>A) has
Q34: In order for a reaction to occur,
Q35: An endothermic reaction is one that<br>A) releases
Q36: Which of the following biological molecules are
Q37: One serving of animal crackers (30 g)contains
Q38: How is a biochemical pathway different than