Multiple Choice
Which of the following reactions releases the smallest amount of heat? 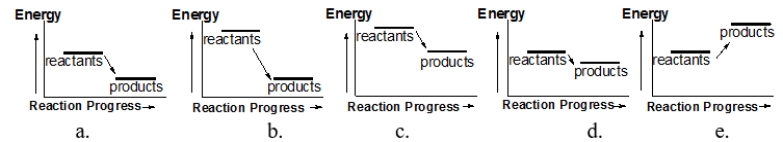
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: The energy difference between I and II
Q51: The first law of thermodynamics states that<br>A)
Q52: What is a biochemical pathway?<br>A) the path
Q53: How does increasing the temperature of a
Q54: The reaction between acetic acid (CH<sub>3</sub>COOH)and methanol
Q56: One serving (45 g)of dry wild rice
Q57: The name for the heat energy released
Q58: How many calories are in a food
Q59: An overview of metabolism is shown in
Q60: In an exothermic reaction, the reactants are