Multiple Choice
Which statement BEST describes how the temperature of the surroundings changes as a result of the reaction represented by energy diagram below? 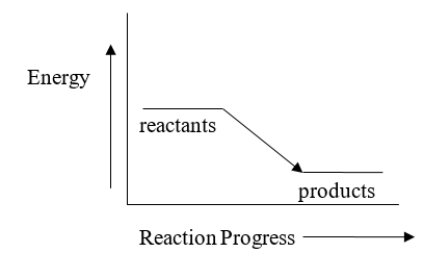
A) The temperature of the surroundings does not change.
B) The temperature of the surroundings increases.
C) The temperature of the surroundings decreases.
D) The surroundings will become very cold.
E) It is not possible to predict anything about the temperature of the surroundings based on this diagram.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which statement BEST describes how calorimetry is
Q2: An endothermic reaction absorbs heat when it
Q3: A calorimeter is used to measure the
Q5: The following reaction is a reversible reaction.Which
Q6: When bonds break, energy is<br>A) always released.<br>B)
Q7: Which of the following changes increases the
Q8: Select the TRUE statement concerning rate of
Q9: Convert 3.5 × 10<sup>4</sup> J to calories.<br>A)
Q10: Why does increasing the concentration of reactants
Q12: If a reaction has a ΔH<sub>rxn</sub> >