Multiple Choice
What is the balanced chemical equation for the reaction illustrated in Figure I? 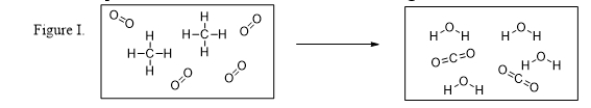
A) CH4 + 2 O2 → 2 H2O + CO2
B) CH4 + O2 → H2O + CO2
C) 2 CH4 + 4 O2 → 4 H2O + 2 CO2
D) 2 C + 8 H + 8 O → 2 C + 8 H + 8 O
E) C + 4 H + 4 O → C + 4 H + 4 O
Correct Answer:

Verified
Correct Answer:
Verified
Q50: Coefficients are important components of chemical equations.What
Q51: How is a nuclear reaction different from
Q52: Which statement BEST describes the following reaction?
Q53: Acetylene (C<sub>2</sub>H<sub>2</sub>)is a small organic molecule used
Q54: Which of the following transformations is a
Q56: Which of these figures illustrate chemical reactions?
Q57: The following reaction is an example of
Q58: The ionic compound CaCO<sub>3</sub> has a formula
Q59: How many copper atoms are in 0.5
Q60: Which of the following is NOT an