Multiple Choice
What type of reaction is the addition of hydrochloric acid (HCl) to propene? 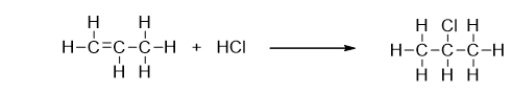
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: The balanced chemical equation for the combustion
Q33: How many atoms of oxygen are present
Q34: Is methane (CH<sub>4</sub>)undergoing oxidation or reduction during
Q35: A chemical reaction involves many changes but
Q36: Which statement describes how molecular mass and
Q38: Which of the following is NOT indicated
Q39: Which of the following correctly determines the
Q40: Does one mole of the antibiotic penicillin
Q41: Calculate the mass of one mole of
Q42: A solution of glucose has 10.00 g