Multiple Choice
Does formaldehyde have a permanent dipole? 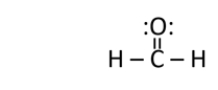
A) Yes.The carbon is partially negative, and the oxygen is partially positive.
B) Yes.The carbon is partially negative, and the hydrogen is partially positive.
C) Yes.The carbon is partially positive, and the oxygen is partially negative.
D) Yes.The carbon is partially positive, and the hydrogen is partially negative.
E) No.Formaldehyde only has a temporary dipole.
Correct Answer:

Verified
Correct Answer:
Verified
Q43: Which element is the LEAST electronegative?<br>A) potassium<br>B)
Q44: How many electron groups are found on
Q45: In molecules with permanent dipoles, _ are
Q46: Nitrogen trichloride, once used as a bleaching
Q47: Which of the following molecules contains polar
Q49: Which of the molecules below does NOT
Q50: What is the molecular geometry of the
Q51: An atom in a molecule has one
Q52: Which of the following steps is done
Q53: Ethylene is used as a starting material