Multiple Choice
Ethanol is an alcohol found in wine and beer, and it is also used as a fuel.It has the structure shown below.What is the strongest type of intermolecular interaction attracting molecules of ethanol together? 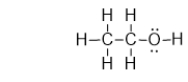
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
Correct Answer:

Verified
Correct Answer:
Verified
Q1: In addition to single bonds, which of
Q2: Which of the bonds in the following
Q3: What is the electron geometry of each
Q5: What is meant by the following symbols?
Q6: What is the electron geometry of the
Q7: The electronegativity difference between C and O
Q8: What is the molecular geometry of each
Q9: How is estradiol recognized by the estrogen
Q10: Which elements have the highest electronegativity and
Q11: What is the molecular geometry of carbon