Multiple Choice
Is the aldehyde oxidized or reduced during the following reaction, and how can you tell? 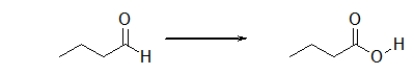
A) Neither.This is not an oxidation-reduction reaction.
B) The aldehyde is neither oxidized nor reduced because the number of hydrogens does not change.
C) The aldehyde is oxidized because it loses two hydrogens.
D) The aldehyde is reduced because it loses two hydrogens.
E) The aldehyde is oxidized because it gains an oxygen.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Is the alkene oxidized or reduced during
Q18: What is the product of the following
Q19: The following reaction occurs in the body.Which
Q20: What is the name of the functional
Q22: When ethanol is metabolized, it is<br>A) synthesized.<br>B)
Q23: Which molecule can be oxidized to a
Q24: What is the product when this compound
Q25: Consumption of ethanol represents "empty calories" because<br>A)
Q26: What is the product of the following
Q84: Which of the following statements describes the