Multiple Choice
A common misconception is that the following molecule contains two functional groups, an alcohol and a ketone, but this is not the case.What functional group does this molecule actually contain, and why is it important to differentiate between this functional group and an alcohol? 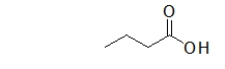
A) This molecule contains a carboxylic acid, which reacts very differently than an alcohol.
B) This molecule contains a carboxylic acid, which is an isomer of an alcohol.
C) This molecule contains a carbonyl, which hydrogen bonds much more than an alcohol.
D) This molecule contains a carbonyl, which reacts very differently than an alcohol.
E) This molecule contains a carboxylic acid, which is unreactive.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which molecule is ethyl acetate?<br>A) CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub><br>B) CH<sub>3</sub>CH<sub>2</sub>COOCH<sub>3</sub><br>C)
Q3: Which biomolecule contains three ester functional groups?<br>A)
Q4: Which molecules contain a carboxylic acid?<br>A) CH<sub>3</sub>COOH<br>B)
Q5: Which of the following molecules contains at
Q6: Which statement BEST describes the role of
Q7: Which molecule contains an amide?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7343/.jpg"
Q8: Which molecule is acetic acid?<br>A) CH<sub>3</sub>COOH<br>B) CH<sub>3</sub>COO<sup>-</sup><br>C)
Q9: Which molecules contain a carboxylic acid?<br>A) HOCH<sub>2</sub>CHO<br>B)
Q10: Below are several different molecules.Which ones contain
Q11: Which molecule is cyclohexanone? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7343/.jpg" alt="Which