Multiple Choice
Do you expect a soap molecule to be more or less soluble than a carboxylic acid with the same number of carbons shown below? 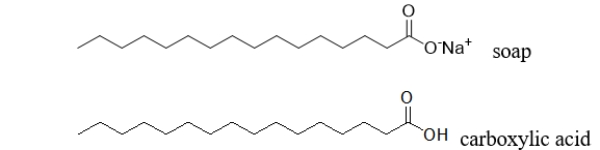
A) less soluble because the soap has a higher molecular weight
B) more soluble because the soap has a higher molecular weight
C) less soluble because the soap is more polar
D) less soluble because the soap is less polar
E) more soluble because the soap is negatively charged
Correct Answer:

Verified
Correct Answer:
Verified
Q15: What is the IUPAC name of the
Q16: Which statement about amides is FALSE?<br>A) The
Q17: What is the strongest intermolecular force of
Q18: What type of biomolecule is shown below?
Q19: What is the name of this ester?
Q21: What is the role of dopamine in
Q22: The portion of the molecule labeled I
Q23: Glucose, the most common sugar, contains which
Q24: Which molecule is butanal? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7343/.jpg" alt="Which
Q25: Which BEST describes the functional group(s)in this