Multiple Choice
The diagram shows three isotherms for an ideal gas, with T3-T2 the same as T2-T1.It also shows five thermodynamic processes carried out on the gas.Rank the processes in order of the change in the internal energy of the gas, least to greatest. 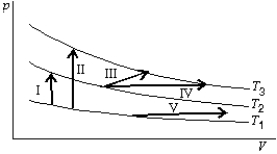
A) I, II, III, IV, V
B) V; then I, III and IV tied; then II
C) V; I; then III, and IV tied; then II
D) II; then I, III and IV tied; then V
E) II; I; then III, IV, and V tied
Correct Answer:

Verified
Correct Answer:
Verified
Q3: A gas is confined to a cylindrical
Q30: Energy transferred into an ideal gas as
Q36: The mean free path of molecules in
Q44: How many molecules are in a sample
Q72: A real gas undergoes a process which
Q79: An ideal gas of N diatomic molecules
Q82: The pressure of an ideal gas is
Q106: The temperature of n moles of
Q111: The ratio of the specific heat of
Q112: A system consists of N gas molecules,