Multiple Choice
In the energy level diagram shown in the figure below, the electron is excited to the E4 energy level.If the electron transitions to an energy level giving off a photon, which level would produce a photon with the largest wavelength? 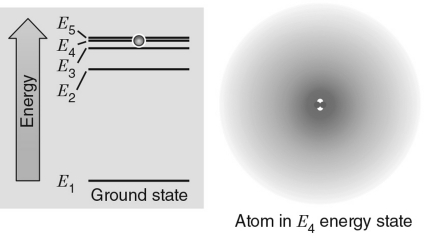
A) E1
B) E2
C) E3
D) E4
E) E5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: If you find that the hydrogen alpha
Q56: How are atoms excited, and why do
Q57: Light has aspects of<br>A) only a wave.<br>B)
Q58: The first five energy levels of hydrogen
Q59: As a blackbody's temperature increases, it also
Q61: When an electron moves from a higher
Q62: As an object's temperature increases, the atoms
Q63: The color of visible light is determined
Q64: In the quantum mechanical view of the
Q65: Imagine you observed three different stars: a