Multiple Choice
In the energy level diagram shown in the figure below, the electron is excited to the E2 energy level.If the atom absorbs a photon with the exact frequency to move the electron to another energy level, which energy level would correspond to the incoming photon with the largest frequency? 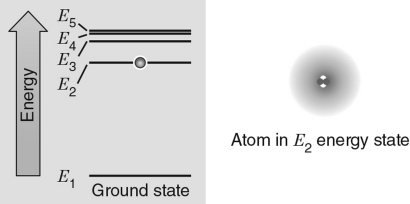
A) E1
B) E2
C) E3
D) E4
E) E5
Correct Answer:

Verified
Correct Answer:
Verified
Q26: If you want a blackbody's peak wavelength
Q27: Compare the differences between a photon of
Q28: Which of the following photons carries the
Q29: What wavelengths of light can the
Q30: Saying that something is quantized means that
Q32: If Jupiter has a temperature of 165
Q33: The frequency of a wave is<br>A) the
Q34: _ measured the speed of light using
Q35: Which of the following lists different types
Q36: The term quantum refers to<br>A) anything that