Multiple Choice
Scandium, 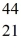 Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?
Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?
A) 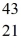 Sc
Sc
B) 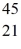 Sc
Sc
C) 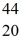 Ca
Ca
D) 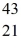 Ca
Ca
E) 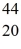 Sc
Sc
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: A radioactive nuclide of atomic number Z
Q20: Going from medium mass nuclei to heavy
Q24: <sub>An isotope of Tc having a half-life
Q31: A 70-kg laboratory technician absorbs 2.9 mJ
Q31: Plutonium-239 decays into uranium-235 plus an alpha
Q50: If a nucleus decays by β<sup>- </sup>decay
Q54: The half-life of cobalt-60 is 5.3 years,while
Q56: If a nucleus had a diameter of
Q60: An air sample is contaminated with <sup>15</sup>O,which
Q78: The unstable isotope <sup>234</sup>Th decays by β