Multiple Choice
A collection of atoms has 20% of the sample in a state 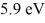 above the ground state. If these emit coherent radiation, what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
above the ground state. If these emit coherent radiation, what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 210 nm
B) 91 nm
C) 340 nm
D) 34 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: What is the minimum speed needed by
Q10: If two electrons in the same atom
Q19: What is the electron configuration for ground
Q24: An electron in a hydrogen atom has
Q29: In a ruby laser,an electron jumps from
Q29: Which of the following are characteristics of
Q34: An atom with 5 electrons is in
Q36: If the principal quantum number of an
Q38: What is the correct electronic configuration for
Q48: An electron in a hydrogen atom has