Multiple Choice
Upon being struck by 240-nm photons, a metal ejects electrons with a maximum kinetic energy of 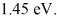 What is the work function of this metal? (h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J)
What is the work function of this metal? (h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J)
A) 3.73 eV
B) 3.13 eV
C) 4.33 eV
D) 4.92 eV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: When a metal surface is illuminated with
Q6: A photon of wavelength 18.0 pm is
Q12: Light excites atomic hydrogen from its lowest
Q26: The energy of the ground state in
Q27: In a double slit experiment,a beam of
Q31: The Bohr radius of the hydrogen atom
Q37: A metal having a work function of
Q43: Gamma rays are photons with very high
Q44: A nonrelativistic electron has a kinetic energy
Q44: Calculate the kinetic energy (in eV) of