Multiple Choice
What is the orbital radius of the 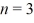 excited state in the Bohr model of the hydrogen atom? The ground-state radius of the hydrogen atom is 0.529 × 10-10 m.
excited state in the Bohr model of the hydrogen atom? The ground-state radius of the hydrogen atom is 0.529 × 10-10 m.
A) 0.477 nm
B) 0.159 nm
C) 0.382 nm
D) 0.549 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: X-rays of energy 2.9 × 10<sup>4</sup> eV
Q9: A beam of red light and a
Q9: A hydrogen atom makes a downward transition
Q11: In a particular case of Compton scattering,a
Q13: A photon of initial wavelength 0.651 nm,after
Q14: A nonrelativistic electron is accelerated from rest
Q17: In a photoelectric effect experiment,electrons emerge from
Q33: Light of wavelength 400 nm falls on
Q39: A hydrogen atom initially in the n
Q42: A metal having a work function of