Multiple Choice
A hydrogen atom is in its n = 2 excited state when its electron absorbs a photon of energy 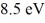 . What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
. What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 5.1 eV
B) 6.6 eV
C) 6.9 eV
D) 7.7 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q9: A hydrogen atom makes a downward transition
Q10: A metal surface has a work function
Q14: A nonrelativistic electron is accelerated from rest
Q16: A single slit is illuminated at normal
Q17: In a photoelectric effect experiment,electrons emerge from
Q18: A photon of wavelength 29 pm is
Q19: An 84-kW AM radio station broadcasts at
Q22: A gas of helium atoms (each of
Q33: Light of wavelength 400 nm falls on
Q37: How fast must a nonrelativistic electron move