Multiple Choice
Assuming the radius of diatomic molecules is approximately 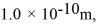 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is 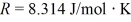 = 0.0821 L ∙ atm/mol ∙ K.
= 0.0821 L ∙ atm/mol ∙ K.
A) 4.9 × 10-8 atm
B) 6.9 × 10-8 atm
C) 1.5 × 10-7 atm
D) 2.2 × 10-7 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q4: If we double the root-mean-square speed (thermal
Q8: The mean free path of an oxygen
Q11: As a result of any natural process,the
Q14: What is the average kinetic energy of
Q21: An ideal gas is kept in a
Q25: The mean free path of an oxygen
Q33: At 50.0°C,the average translational kinetic energy of
Q34: An ice cube at 0°C is placed
Q50: A container is filled with a mixture
Q54: Eleven molecules have speeds 16,17,18,...,26 m/s.Calculate the