Multiple Choice
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 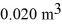 to
to 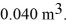 The final pressure is
The final pressure is 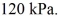 The ideal gas constant is R = 8.314 J/mol ∙ K. The heat transfer to the gas is closest to
The ideal gas constant is R = 8.314 J/mol ∙ K. The heat transfer to the gas is closest to
A) 3.3 kJ.
B) 1.7 kJ.
C) -3.3 kJ.
D) -1.7 kJ.
E) 0.00 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: When a fixed amount of ideal gas
Q12: Two metal rods, one silver and the
Q13: A radiating body originally has a Kelvin
Q13: A person pours 330 g of water
Q14: During an isothermal process,5.0 J of heat
Q17: A heat conducting rod, 1.40 m long,
Q19: A blacksmith is flattening a steel plate
Q27: A chunk of ice (T = -20°C)is
Q42: An ideal gas initially at 300 K
Q52: A cylinder contains 23 moles of an