Multiple Choice
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell, use the partial activity series included to identify the components labeled 1, 2, 3, and 4 respectively. 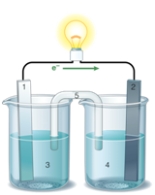 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) iron, nickel, iron ion solution, nickel ion solution
B) nickel, iron, iron ion solution, nickel ion solution
C) nickel, iron, nickel ion solution, iron ion solution
D) iron, nickel, nickel ion solution, iron ion solution
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The oxidation half-reaction for the reaction
Q2: Write the balanced net ionic equation
Q9: Which example does NOT illustrate a redox
Q13: What is the oxidation number of carbon
Q18: Given the accompanying partial activity series,predict
Q19: What is the oxidation number of sulfur
Q22: What is the oxidation number of sulfur
Q24: What are the coefficients of the
Q32: Given the accompanying partial activity series,which
Q35: Which pair of half-reactions takes place